CLINICAL RESEARCH
Investigating
the Right Dose,
the Right Place,
the Right Amount of Time
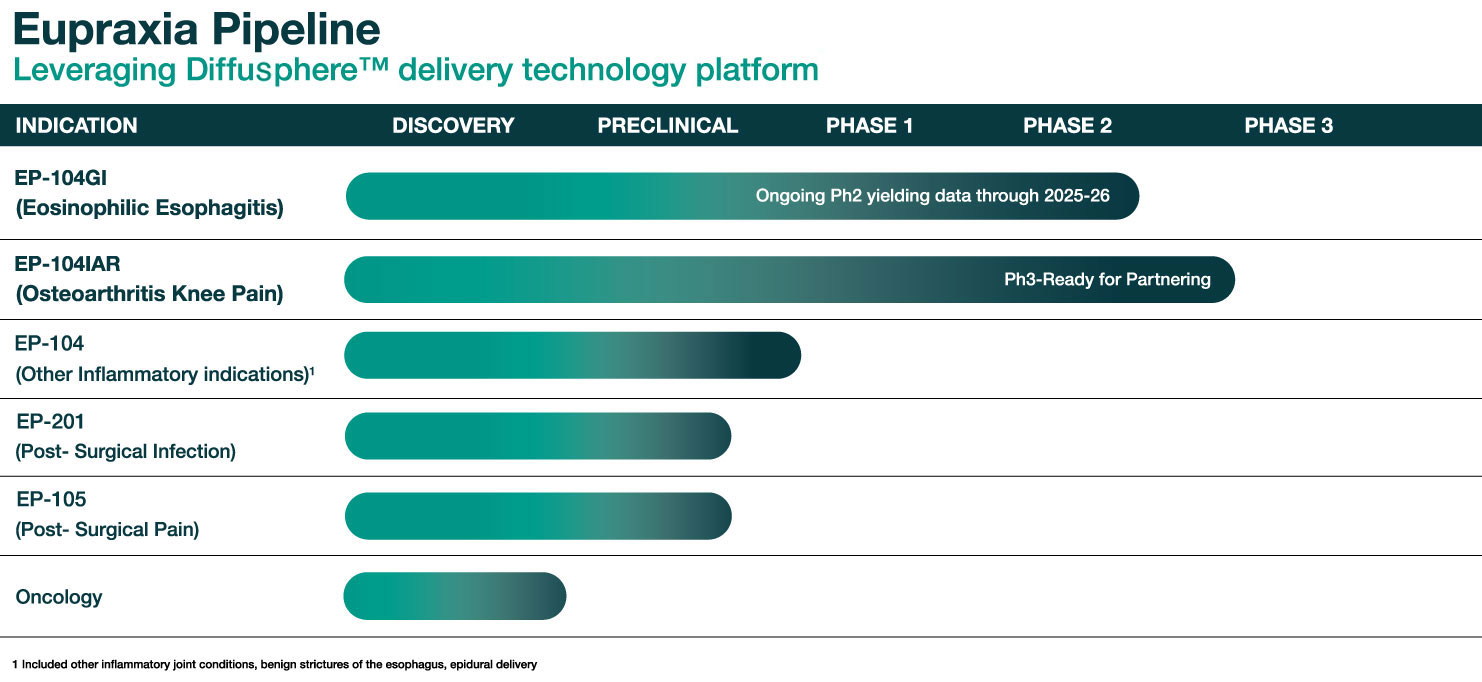
Eupraxia is currently pursuing the clinical development of EP-104GI for the treatment of eosinophilic esophagitis (EoE) and EP-104IAR for knee osteoarthritis (OA).
We are also exploring the application of the DiffusphereTM drug delivery technology to other active compounds to address unmet needs in other inflammatory diseases, pain conditions and oncology indications.
RESOLVE: A Phase 1b/2 Trial in Eosinophilic Esophagitis (ONGOING)
RESOLVE (NCT05608681) is a Phase 1b/2, multicenter, open-label, dose-escalation and optimization trial evaluating the safety, tolerability, feasibility, pharmacokinetics, and efficacy of EP-104GI in adults with EoE.
In the Phase 1b portion, EP-104GI is administrated a single time inside the esophagus tissue during endoscopy in increasing levels (number of sites and dose per site). Participants will be followed for either 24 or 52 weeks after administration.
In the Phase 2 portion, the safety, tolerability and efficacy of single administrations of two doses EP-104GI will be tested against a placebo for up to 52 weeks, with a key primary endpoint assayed after 24 weeks.
SPRINGBOARD: A Phase 2 Trial in Knee Osteoarthritis (COMPLETED)
SPRINGBOARD (NCT04120402) was a randomized, vehicle-controlled, double-blind, phase 2 trial evaluating the safety, tolerability and efficacy of EP-104IAR in Knee OA.
SPRINGBOARD studied the effect on knee pain of a single EP-104IAR injection against a placebo, as well as its safety and tolerability for patients.