OUR SCIENCE
Eupraxia’s proprietary Diffusphere™ technology is designed to deliver medications directly to targeted tissues, aiming to provide sustained therapeutic levels while minimizing systemic exposure*.
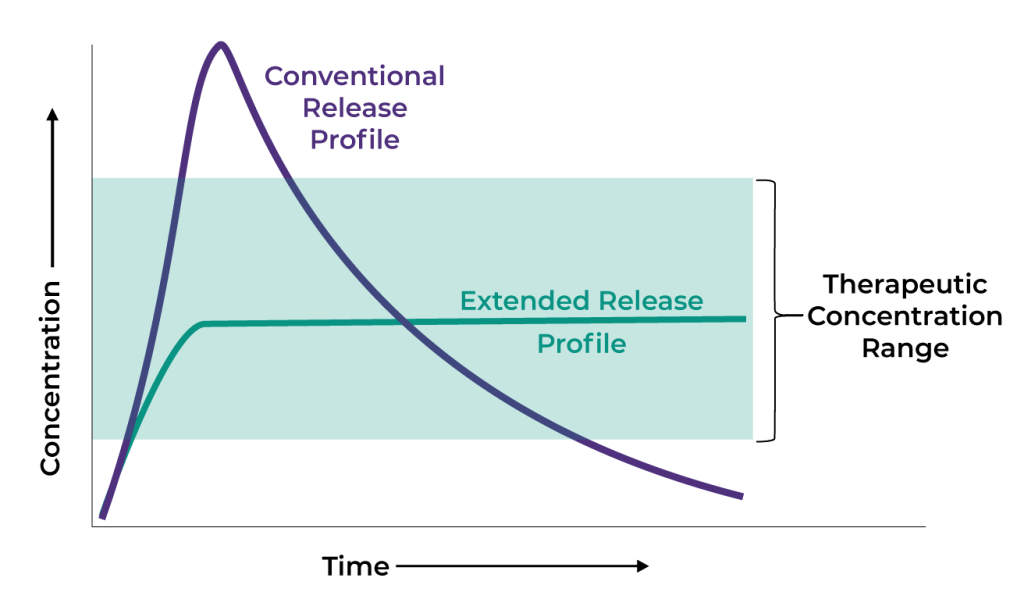
Our Diffusphere™ Technology
Imprecise drug delivery often limits a drug’s safety and efficacy. Conventional delivery exposes both healthy and disease tissues, increasing side effects and reducing efficacy. Frequent dosing is often required to manage this issue, increasing patient burden.
Eupraxia’s proprietary Diffusphere™ technology aims to improve therapies by delivering the right dose of drug, in the right place and for the right duration.
Diffusphere coats an active compound with a micron-thin polymer membrane designed to release this drug core at a defined, constant rate as it dissolves.
Other extended-release approaches mix a small amount of drug in a large polymer matrix. The drug then often releases unevenly as the polymer dissolves, potentially reducing tolerability and efficacy.
With a more controlled, local release, Diffusphere aims to improve therapies duration, efficacy and side effects profile.
Our Clinical Candidates
EP-104GI
A long acting, controlled-release fluticasone propionate formulation designed to be injected in the esophagus tissue, in development for the treatment of Eosinophilic Esophagitis (EoE)
EP-104IAR
A long acting, controlled-release fluticasone propionate formulation designed to be injected in the intraarticular space, in development for the treatment of Knee Osteoarthritis (OA)
Eupraxia is developing EP-104GI for the treatment of Eosinophilic Esophagitis
What is Eosinophilic Esophagitis (EoE)?*
In EoE, inflammatory white blood cells infiltrate the esophagus, leading to:
Pain and difficulty swallowing
Food stuck in the esophagus, necessitating emergency visits
Anxiety and depression
Long-term risk of esophageal remodeling of the esophagus and strictures, if left untreated or incompletely treated
EoE has seen a significant Increase In prevalence:*
Global cases have seen a sharp rise in recent years
US prevalence: 1 in 700 individuals (142.5 per 100,000)
Estimated healthcare cost (US, 2024): $1.3 billion
Projected emergency visits by 2030: Over 15,000 annually
Treatment challenges in EoE*
Swallowed corticosteroids used in the treatment of EoE lead to a short contact with the esophagus
Current standards of care in EoE overall result in poor adherence, with over 40% of patients non-compliant with their treatment
EP-104GI in EoE
EP-104GI, as a long-acting submucosal corticosteroid formulation to be injected within esophageal tissues has the potential to increase patient adherence by requiring fewer interventions, while addressing local inflammation.
EP-104GI is currently studied in the RESOLVE Phase 1b/2 clinical trial.
Eupraxia is developing EP-104IAR for the treatment of pain in Knee Osteoarthritis
Osteoarthritis is the leading cause of disability in older adults* and knee OA alone affects more than 30 million people in the US *. This includes 18 million who suffer from knee pain or some form of disability. Knee OA can severely impact quality of life through reduced mobility, depression and loss of sleep*.
The American College of Rheumatology strongly recommends corticosteroids for the treatment of knee OA pain*.
Although they reduce pain for a short duration, current corticosteroids can result in systemic side effects, such as glucose levels increase or adrenal suppression*. This especially limits their use in patients with diabetes or who require treatment in more than one joint.
EP-104IAR is designed to release corticosteroids locally, over an extended period, reducing systemic exposure and potentially limiting side effects. This may result in pain relief over an increased duration, with an improved safety profile over current options, including in at-risk populations.
